Psy-Fer / Squigglekit
Programming Languages
Labels
Projects that are alternatives of or similar to Squigglekit
SquiggleKit
A toolkit for accessing and manipulating nanopore signal data
Full documentation: https://psy-fer.github.io/SquiggleKitDocs/
publication: SquiggleKit: A toolkit for manipulating nanopore signal data
Pre-print: SquiggleKit: A toolkit for manipulating nanopore signal data
Coming changes
Fast5_fetcher: merge single files into multi-fast5 files
SquigglePull: python3, read from multi-fast5
SquigglePlot: python3, read from multi-fast5, image size args, arg clean-up
Segmenter: dynamic file formats and more stability
MotifSeq: Improved background modelling, custom modelling, RNA specific tools, custom alignment methods
Overview
| Tool | Category | Description |
|---|---|---|
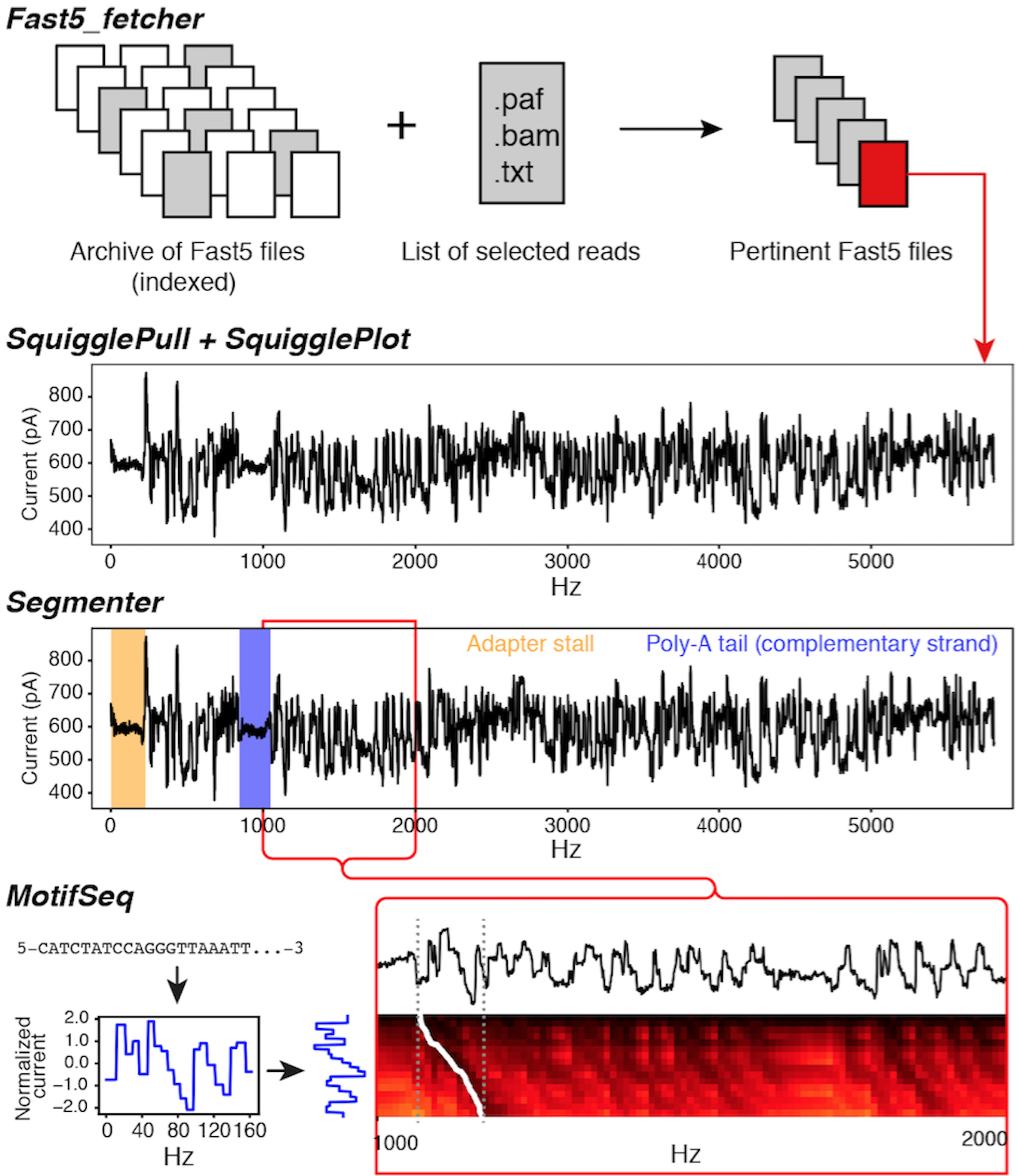
| Fast5_fetcher | File management |
Fetches fast5 files given a filtered input list |
| SquigglePull | Signal extraction |
Extracts event or raw signal from data files |
| SquigglePlot | Signal visualisation |
Visualisation tool for signal data |
| Segmenter | Signal analysis |
Finds adapter stall, and homopolymer regions |
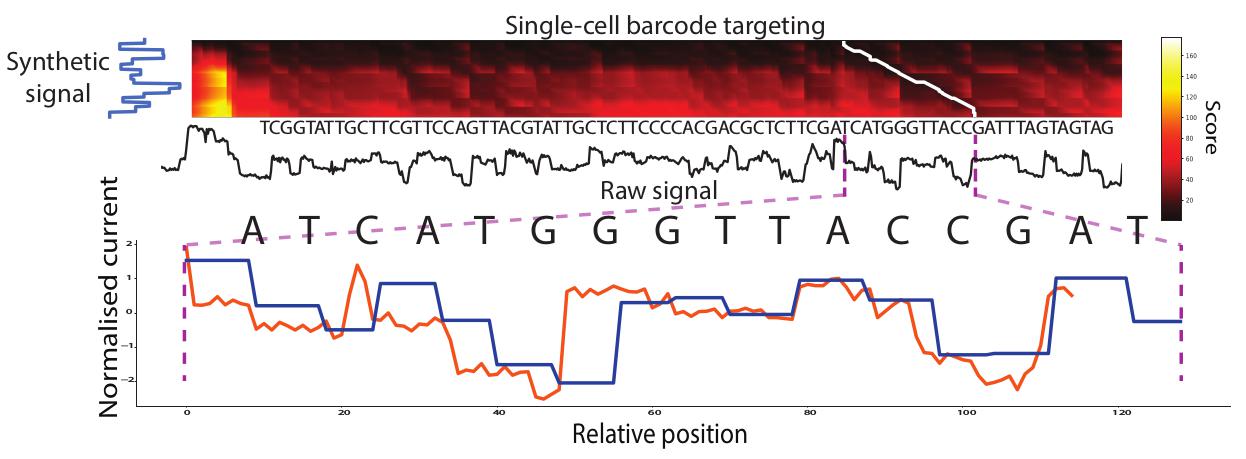
| MotifSeq | Signal analysis |
Finds nucleotide sequence motifs in signal, i.e.“Ctrl+F” |
Requirements
Following a self imposed guideline, most things written to handle nanopore data or bioinformatics in general, will use as little 3rd party libraries as possible, aiming for only core libraries, or have all included files in the package.
In the case of fast5_fetcher.py and batch_tater.py, only core python libraries are used. So as long as Python 2.7+ is present, everything should work with no extra steps.
There is one catch. Everything is written primarily for use with Linux. Due to MacOS running on Unix, so long as the GNU tools are installed (see below), there should be minimal issues running it. Windows however may require more massaging. The Windows-Subsystem-Linux must be installed. Follow the instructions here to do this.
SquiggleKit tools were not made to be executable to allow for use with varying python environments on various operating systems. To make them executable, add #! paths, such as #!/usr/bin/env python2.7 as the first line of each of the files, then add the SquiggleKit directory to the PATH variable in ~/.bashrc, export PATH="$HOME/path/to/SquiggleKit:$PATH"
Install
git clone https://github.com/Psy-Fer/SquiggleKit.git
Use pip for python 2 and pip3 for python 3. User environments may vary.
for
fast5_fetcher.py, SquigglePull.py, segmenter.py:
- numpy
- matplotlib
- h5py
- sklearn
- ont_fast5_api
pip install numpy h5py sklearn matplotlib
for MotifSeq.py:
- all of the above
- scipy
- scrappie
- mlpy 3.5.0 (only use pip3 in python 3 - see below)
pip install scipy scrappie
Installing mlpy:
Python2
- Download the Files
- Install Instructions
Python3.6 (Python3.7 not working because of a cython/gsl issue)
pip3 install machine-learning-py
Quick start
fast5_fetcher
If using MacOS, and NOT using homebrew, install it here:
homebrew installation instructions
then install gnu-tar with:
brew install gnu-tar
Building the index (not required for multi_fast5)
How the index is built depends on which file structure you are using. It will work with both tarred and un-tarred file structures. Tarred is preferred. (zip and other archive methods are being investigated)
- Raw structure (not preferred)
for file in $(pwd)/reads/*/*;do echo $file; done >> name.index
gzip name.index
- Local basecalled structure
for file in $(pwd)/reads.tar; do echo $file; tar -tf $file; done >> name.index
gzip name.index
- Parallel basecalled structure
for file in $(pwd)/fast5/*fast5.tar; do echo $file; tar -tf $file; done >> name.index
If you have multiple experiments, then cat them all together and gzip.
for file in ./*.index; do cat $file; done >> ../all.name.index
gzip all.name.index
Basic use on a local computer
using a filtered paf file as input:
python fast5_fetcher.py -p my.paf -s sequencing_summary.txt.gz -i name.index.gz -o ./fast5
SquigglePull
All raw data:
python SquigglePull.py -rv -p ~/data/test/reads/1/ -f all > data.tsv
Positional event data:
python SquigglePull.py -ev -p ./test/ -t 50,150 -f pos1 > data.tsv
SquigglePlot
Plot individual fast5 file:
python SquigglePlot.py -i ~/data/test.fast5
Plot files in path
python SquigglePlot.py -p ~/data/ --plot_colour -g
Plot first 2000 data points of each read from signal file and save at 300dpi pdf:
python SquigglePlot.py -s signals.tsv.gz --plot_colour teal -n 2000 --dpi 300 --no_show o--save test.pdf --save_path ./test/plots/
Segmenter
Identify any segments in folder and visualise each one
Use f to full screen a plot, and ctrl+w to close a plot and move to the next one.
python segmenter.py -p ./test/ -v
Stall identification
python segmenter.py -s signals.tsv.gz -ku -j 100 > signals_stall_segments.tsv
MotifSeq
Find kmer motif:
fasta format for model:
my_kmer.fa
>my_kmer_name
ATCGATCGCTATGCTAGCATTACG
find the best match to that k-mer in the signal:
python MotifSeq.py -s signals.tsv -i my_kmer.fa > signals_kmer.tsv
Limitations
k-mer length should not really be below 12nt, below this things get hairy based on modelling
The p-values and hit probabilities provided are based on loose modelling of negative background scores for a number of k-mers. It is currently only modelled on R9.4 model, not R10 or RNA.
Acknowledgements
I would like to thank the members of my lab, Shaun Carswell, Kirston Barton, Hasindu Gamaarachchi, Kai Martin, Tansel Ersavas, Brent Bevear, Jillian Hammond, and Martin Smith, from the Genomic Technologies team from the Garvan Institute for their feedback on the development of these tools.